In 1935, Hans Berger writes in one of his seminal reports on the electroencephalogram (EEG), addressing the controversy surrounding the origin of the then unbelievable electrical potentials recorded by him from the human scalp:
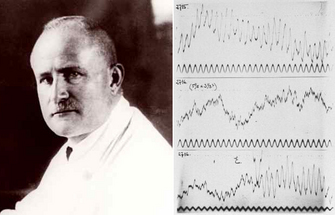 |
| Fig. 1. Hans Berger and his early EEG recordings from the 1930s. Adapted from Wiki Commons. |
"I disagree with the statement of the English investigators that the EEG originates exclusively in the occipital lobe. The EEG originates everywhere in the cerebral cortex...In the EEG a fundamental function of the human cerebrum intimately connected with the psychophysical processes becomes visible manifest." (see here for a history of Hans Berger and the EEG)
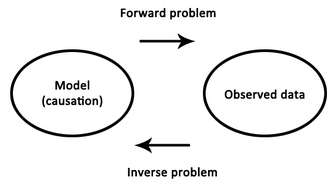 |
| Fig. 2. The forward and inverse problems |
Decades later, the correctness of his position is both a blessing and a curse - we now know that the entire brain produces EEG signals, but it has been a struggle to match components of the EEG to their specific sources in the brain, and thus to further our understanding of how exactly the functioning of the brain relates to those psychophysical processes with which Berger was so enthralled. This struggle is best summarised as an inverse problem, in which one begins with a set of observations (e.g., EEG signals) and has to work backwards to try to calculate what caused them (e.g., neural activity in a specific brain region). A massive obstacle to this approach is the fact that as electrical signals pass from the brain to the scalp they become heavily distorted by the skull. This distortion makes it exceedingly difficult to try to reconstruct the underlying sources in the brain.
In 1969, the journey to understand the electrical potentials of the brain took an interesting and fruitful detour when David Cohen, a physicist working at MIT, became the first to confidently measure the incredibly tiny magnetic fields produced by the heart's electrical signals (see here for a talk by David Cohen on the origins of MEG). To do this, he constructed a shielded room, blocking interference from the overwhelming magnetic fields generated by earth itself and by other electrical devices in the vicinity, effectively closing the door on a cacophony of voices to carefully listen to a slight
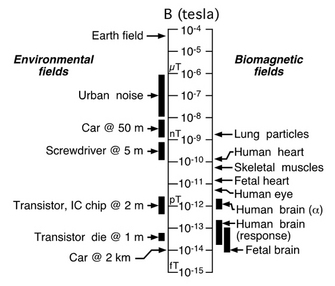 |
| Fig. 3. Comparisons of magnetic field strengths on a logarithmic scale. From Vrba (2002). |
This approach to record the brain's magnetic fields, rather than the electrical potentials themselves, was advanced even further by James Zimmerman and others working at the Ford Motor Company, where they developed the SQUID, a superconducting quantum interference device. A SQUID is an extremely sensitive magnetometer, operating on the principles of quantum physics beyond the scope of this article, which is able to detect precisely those very tiny magnetic fields produced by the brain. To appreciate the contributions of magnetic shielding and SQUIDs to magnetoencephalography, consider that the earth's magnetic field, the one acting on your compass needle, is at least 200 million times the strength of the fields generated by your brain trying to read that very same
compass.
compass.
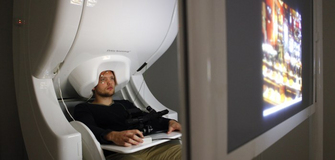 |
| Fig. 4. A participant being scanned inside a MEG scanner. From OHBA. |
Given that these magnetic fields occur simultaneously with electrical activity, MEG is afforded the same millisecond resolution as EEG, allowing one to examine neural activity at its natural temporal resolution. This is in contrast to functional magnetic resonance imaging, fMRI, which, using magnetic fields as a tool rather than a target of measurement, actually measures changes in blood oxygenation which occur on the order of seconds, making it impossible to effective pinpoint the time of neural activity (see here). Another advantage over fMRI is the fact that electromagnetic signals are more directly related to the underlying neural activity than the haemodynamic response, which may differ across brain regions, clinical populations, or with respect to drug effects, thereby complicating interpretations of observed effects. Unlike the electrical potentials measured in EEG, however, the magnetic fields measured in MEG pass from the brain through the skull in a relatively undisturbed manner, substantially simplifying the inverse problem. In these ways, for a non-invasive technique, MEG best combines high temporal resolution and improves source localisation within the human brain.
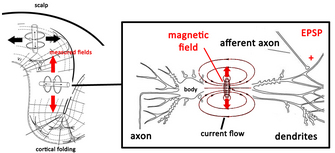 |
| Fig. 5. The source of recorded magnetic fields in MEG. Adapted from Hansen et al. (2010) |
At the end of a long and arduous MEG scanning session, one is left with about 300 individual time series, typically recorded at 1000 Hz, reflecting tiny changes in magnetic fields driven by neural activity presumably occuring in response to some cognitive task. Although the shielded room blocks out magnetic interference from other electrical devices (and all equipment inside the room works through optical fibres), there is still massive interference from the subject's heart and any other muscle activity around the head. For this reason, participants are typically instructed to limit eye movements and blinking and any remaining artefactual noise in the data (i.e., anything not thought to be brain activity) is taken out at the analysis stage using techniques like independent component analysis.
 |
| Fig. 6. Raw MEG data (left), and event-related fields in sensor space and source space (right). Adapted from Schoenfeld et al. (2014). |
Analysis of MEG data can be done in sensor space, in which one simply looks at how the signals at individual sensors change during different parts of a cognitive task. This provides a rough estimate of the activation patterns along the cortex. The perk of MEG, however, is the ability to project data recorded in the 300 sensors to source space, and effectively estimate where in the brain these signals may originate. Although this is certainly more feasible in MEG than EEG, the inverse problem is actual a fundamental issue to both types of extracranial recordings (we don't have this problem when measuring directly from the brain during intracranial recording). One way to narrow down which possible activation regions in the brain could underlie the observed magnetic fields is to establish certain assumptions about what we expect brain activity to look like in general, and how that activity is translated into the signal measured at the scalp. Such assumptions are more reasonable in MEG than EEG due to the higher fidelity of magnetic fields as they pass from the brain to scalp.
 |
| Fig. 7. Neural activation is smooth, forming clusters of active neurons. Adapted from Wiki Commons. |
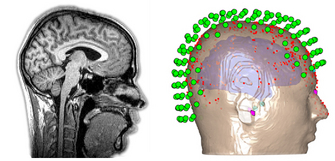 |
| Fig. 8. MRI structural image of the head and brain (left), and sensor, head, and brain model (right). Adapted from Wiki Commons and OHBA. |
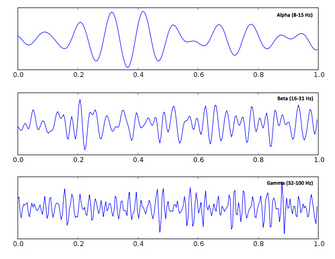 |
| Fig. 9. Alpha, beta, and gamma oscillations. Adapted from Wiki Commons. |
There are two general approaches when analyzing MEG data. Analysis of event-related fields looks at how the timing or the size of the magnetic
Other resources:
For a slightly more in-depth description of MEG, see here.
For a more in-depth description of MEG acquisition, see this video.
References
Baillet, S., Mosher, J.C., & Leahy, R.M. (2001). Electromagnetic brain mapping. IEEE Signal Processing Magazine..
Fernando H, Lopes da Silva. MEG: an introduction to methods. eds: Hansen, Kringelback & Salmelin. USA: OUP, 2010:1-23, figure 1.3 from p6.
La Vaque, T. J. (1999). The History of EEG Hans Berger: Psychophysiologist. A Historical Vignette. Journal of Neurotherapy.
Proudfoot, M., Woolrich, M.W., Nobre, A.C., & Turner, M. (2014). Magnetoencephalography. Pract Neurol, 0, 1-8.
Schoenfeld, M.A., Hopf, J-M., Merkel, C. Heinz, H-J., & Hillyard, S.A. (2014). Object-based attention involves the sequential activation of feature-specific cortical modules. Nature Neuroscience, 17(4).
Vrba, J. (2002). Magnetencephalography: the art of finding a needle in a haystack. Physica C, 368, 1-9.
The data figures are from papers cited above. All other figures are from Wiki Commons.






